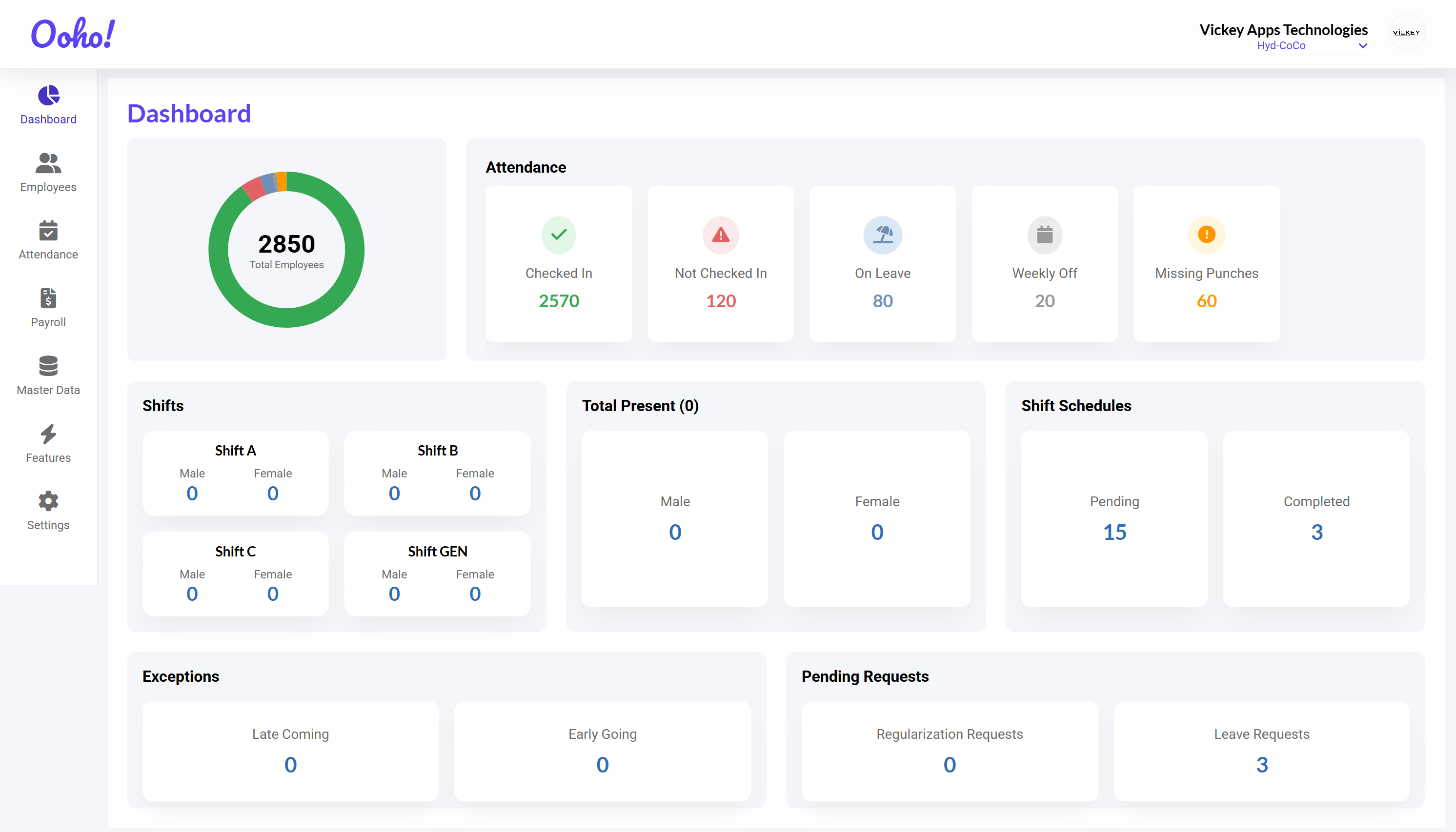
Complete HRMS Solution for
Pharma Companies
GMP-Compliant HR & Payroll Software with Training Management, Shift Scheduling, Regulatory Documentation, and Compliance Tracking. Built for Pharma Excellence.